Washington, DC— Did you know that there are at least 17 crystalline forms of ice, many of them formed under extreme pressures, such as those found in the interiors of frozen planets? New work from a team led by Carnegie’s Timothy Strobel has identified the structure of a new type of ice crystal that resembles the mineral quartz and is stuffed with over five weight percent of energy-rich hydrogen molecules, which is a long-standing Department of Energy goal for hydrogen storage.
The results, published by the Journal of the American Chemical Society, could have implications for the mineralogy of icy planetary bodies as well as for energy storage technology.
As all school children learn, water molecules are made up of two hydrogen atoms and one oxygen atom (H2O). Quartz is made up of one silicon atom and two oxygen atoms (SiO2). There are parallels between several forms of frozen H2O and SiO2. For example, tridymite and cristobalite (both SiO2), which are found in volcanic rocks, resemble two of ice’s 17 different crystal structures.
Scientists have predicted for decades that a form of ice that looks like quartz could be created in the laboratory. But until now, a form of ice with such structural similarity was elusive.
Strobel and his colleagues—Carnegie’s Maddury Somayazulu and Stanislav Sinogeikin, along with Przemyslaw Dera of University of Hawaiʻi at Manoa and Russell Hemley of George Washington University—were able to create this quartz-like ice and use advanced diffraction and spectroscopic tools to determine its structure.
The new compound appears under specific conditions: at about 4,000 times normal atmospheric pressure (400 megapascals) and 44 degrees Fahrenheit (280 kelvin).
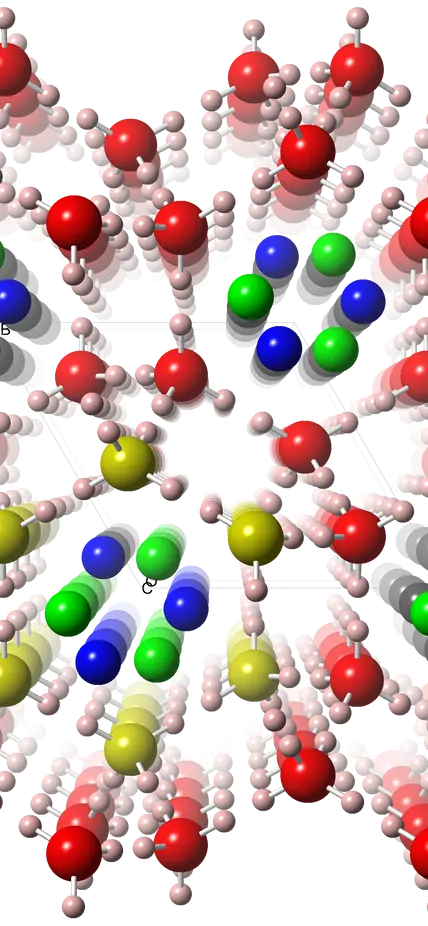
Strobel’s team discovered that its crystalline structure consists of interconnected spiral chains of water molecules that form tubes. From the outside, these tubes look like they’re covered with pentagonal tiles. But on the inside they are filled like manicotti with a corkscrew-like arrangement of hydrogen (H2) molecules.
“While quartz is one of the most abundant minerals found on Earth’s surface, this water-based structure with a similar atomic arrangement might be found in abundance on icy planetary bodies where conditions similar to those under which it was created in our lab might be found,” Strobel said.
And the team’s results have implications beyond planetary science. The compound has a substantial hydrogen content (in the form of H2 molecules) and the large water channels help to enable enhanced H2 mobility. These features may facilitate future practical applications in the area of renewable energy storage.
__________________
This work was supported by EFree, an Energy Frontier Research Center funded by the Department of Energy Basic Energy Sciences.
Portions of this work were performed at the HPCAT Advanced Photon Source at Argonne National Laboratory whose operations are supported by the Department of Energy and instrumentation is partially funded by the National Science Foundation. The portions of the work performed at the Lawrence Livermore National Laboratory were done under the auspices of the Department of Energy.