Baltimore, MD—A team of researchers led by Carnegie Science’s Will Ludington, Karina Gutiérrez-García, and Kevin Aumiller identified genes that enable a beneficial bacterial species to colonize specific regions of the gastrointestinal tract. Their work, published last week in Science, could revolutionize our understanding of how the composition of the gut microbiome is determined and open the door to microbiome engineering.
The gut microbiome is an ecosystem of hundreds to thousands of microbial species living within the human body and influencing our health, fertility, and even longevity. These populations can aid digestion, inform immune responses, and help fight off pathogens among other functions.
However, the microbiome isn’t uniform throughout the gut. Just as various organs within the gastrointestinal system have different specialties when it comes to digesting food and absorbing nutrients, different microbial communities are localized within each zone and play unique roles there.
Successful colonization of each region of the G.I. tract by different microbial populations is dependent on a variety of factors such as nutrient requirements of the bacteria; the local pH and dissolved oxygen content; competition with other bacterial strains; and survivability in harsh conditions—including stomach acid, bile salts, and immune-response cells.
“We’re talking about an incredibly complex system of interconnected microbial communities, and each species needs to get to the right place where it can thrive and contribute to host health,” explained Ludington, who has been probing microbiome acquisition and composition for several years at Carnegie. “Researchers have been trying to figure out how each bacterial species is directed to the right location and how colonization by harmful or less-than-ideal species is minimized.”
Think about checked luggage moving through the system of conveyor belts behind the scenes at a busy, urban airport. The baggage handling system may look disorganized and chaotic, but the majority of bags get to the plane where they need to be. And processes are in place to correct any erroneous sorting that occurs over time.
“Likewise, in the gut, beneficial bacteria need to get to the region where they can successfully create a colony,” co-lead author Gutiérrez-García indicated. “We worked to reveal the mechanisms that enable this to happen.”
Successful colonization hinges on proteins in bacterial cell walls called adhesins. As you might guess from their name, they can stick to a variety of different surfaces within the body. But they typically bind non-specifically, meaning they could just as soon attach to one tissue as another.
So how do symbiotic microbiome species get to the place they need to go?
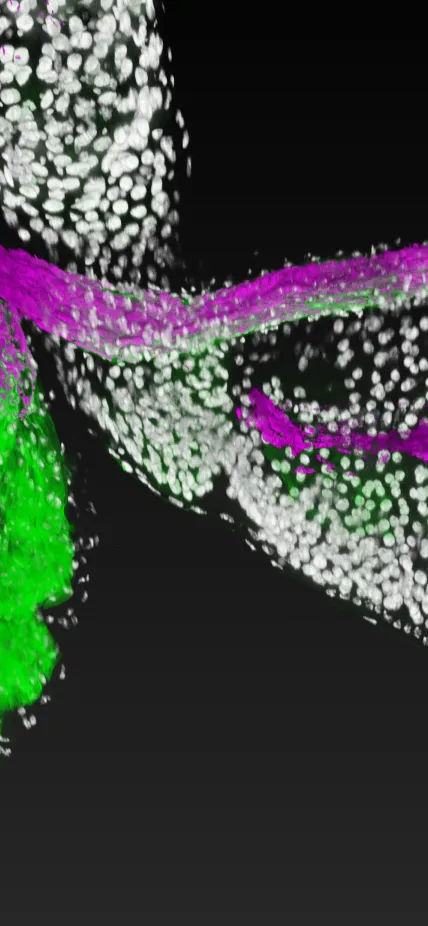
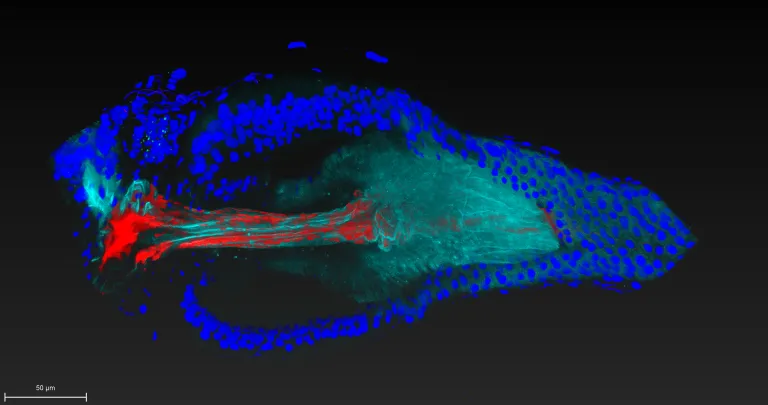
To tackle this mystery, Ludington, Gutiérrez-García, Aumiller and their colleagues developed technology that enabled them to watch a single cell of the bacterial species Lactiplantibacillus plantarum colonize its niche within the fruit fly gut in real time. The team also included Carnegie’s Ren Dodge, Benjamin Obadia, Haolong Zhu, and Ru-Ching Hsia, as well as Ann Deng, Sneha Agrawal, and Xincheng Yuan from Johns Hopkins University and, Richard Wolff and Nandita Garud from UCLA.
The fruit fly may be a pest in the kitchen, but it’s a workhorse in the laboratory and the perfect organism for this type of research, because the species that comprise its microbiome are well defined and small in number.
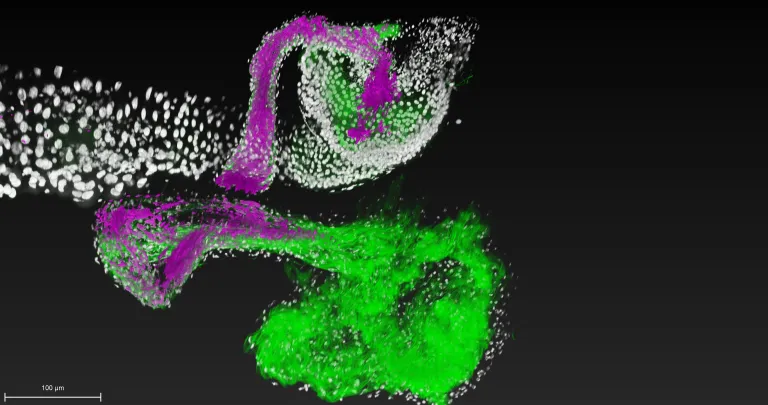
Watching the events unfold in such high-resolution detail enabled the scientists to see the difference between short-lived colonization and long-term success.
“Developing this imaging technique was an exciting challenge,” said Dodge, a key contributor to the study. “It allowed us to see the interactions of individual bacteria cells with the host gut in unprecedented detail.”
They found that L. plantarum isolated from the guts of wild fruit flies was able to stably attach to host tissue whereas L. plantarum from humans and other sources formed only transient attachments.
With this information in hand, the researchers set out to determine the genetic basis for this super-affinity. Through diligent and painstaking work, they were able to identify a set of genes for symbiotic gut colonization within a niche.
“By identifying the genes that enable L. plantarum to colonize specific niches, we now have the insights into how to engineer greater precision into other bacteria,” said Kevin Aumiller, a co-lead author on the project. “This opens the door to creating probiotics that are optimized for specific niches in the human gut.”
“Looking ahead, we will attempt to elucidate the mechanism underlying this binding specificity,” Ludington concluded.
Acknowledgments
This material is based upon work supported by the U.S. National Science Foundation under Awards No. 2032985, 2144342, and 2240098 and the U.S. National Institutes of Health under Awards No. R01DK128454, R21AI173779, R35GM151023, and T32 GM007231.