Research
The Fan Lab studies the molecular mechanisms that govern mammalian development, using the mouse as a model. They use a combination of biochemical, molecular, and genetic approaches to identify and characterize signaling molecules and pathways that control the development and maintenance of the musculoskeletal system.
The musculoskeletal system provides mechanical support for our posture and movement. How it arises during embryogenesis pertains to the basic problem of embryonic induction. How the components of this system are repaired after injury and maintained throughout life is of biological and clinical significance. The Fan Lab studies how this system is generated and maintained.
Current Themes
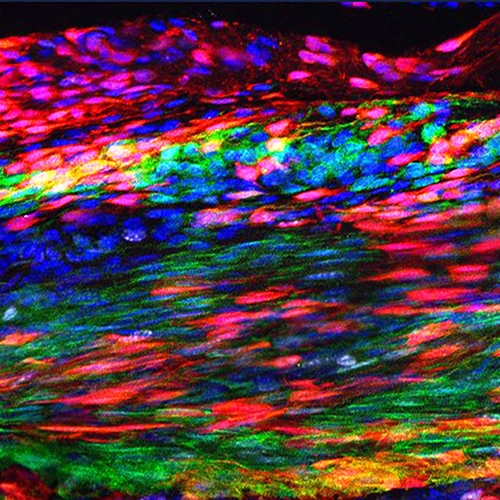
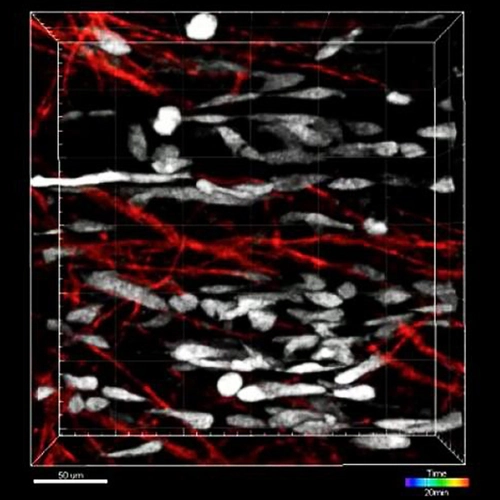
The musculoskeletal system of the trunk originates from a common embryonic structure called the somite. Somites are segmented mesodermal units flanking both sides of the spinal cord. Their reiterated pattern is the basis for the repeated organization of the trunk. Under the inductive influence of adjacent tissues, cells within the somite give rise to muscles and bones. We have developed a 3-dimensional culture system that allows characterization of crucial long-range and contact-dependent cellular interactions that induce early skeletal and muscle fates. Our efforts toward designing new methods and assays to track somite development have enabled us to make novel observations.
Conversely, the Wnt family of proteins plays a key role in inducing the dermis/muscle dual potential progenitors. Combining our in vitro assay with microarrays analyses, we have uncovered previously unknown effectors and target genes of Wnt. Using an ex vivo whole embryo culture system coupled with somite-specific gene delivery, we discovered an unconventional pathway for Wnt signaling via the adenylyl cyclase/protein kinase A/Creb cascade that selectively activates myogenic transcriptional determinants Myf5 and MyoD.
We have identified the Hedgehog (Hh) proteins responsible for inducing the early skeletal fate. Hh largely utilizes evolutionarily conserved downstream mediators for inductive signaling. In addition, we also found a vertebrate-specific cell surface Hh binding protein Gas1. Gas1 mutants display skeletal defects related to or due to altered Hh signaling. Mechanistically, Gas1 helps transform the Hh diffusion gradient into its observed signaling activity gradient. This unexpected mechanism provides a new vision of Hh signaling pathway initiation and has direct implications for the long-range action of Hh.
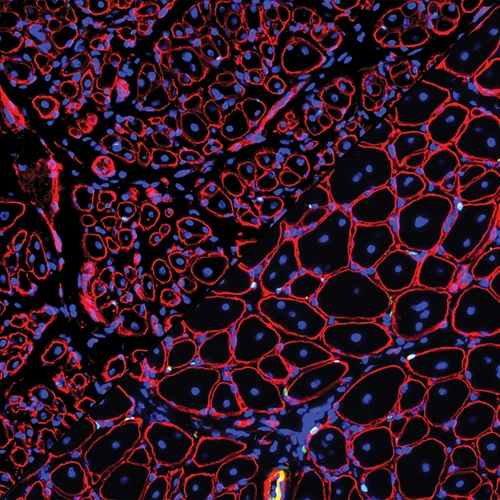
Somites not only supply cells for embryonic muscles, but also contain muscle progenitors. The proliferative capacity of these progenitors depends upon the transcription factors Pax3 and Pax7. Both genes are activated by Wnt. Using inducible cell lineage tracing, we have found that early Pax7-expressing somitic cells directly give rise to adult muscle stem cells, i.e. the satellite cells. Lineage tracing of Pax7-expressing adult satellite cells indicates that they are indeed a stem cell source for muscle regeneration. Conditional inactivation of Pax7 at different developmental time points reveals that Pax7 is required for the proliferative properties of muscle progenitors up to 3 weeks after birth when they transition into quiescence. After this transition is made, however, both Pax3 and Pax7 are completely dispensable. Our finding of an age-dependent cell-intrinsic change in the genetic requirement for muscle stem cells cautions against inferring adult stem cell biology from embryonic studies, and has direct implications for the use of stem cells from hosts of different ages in transplantation-based therapies.