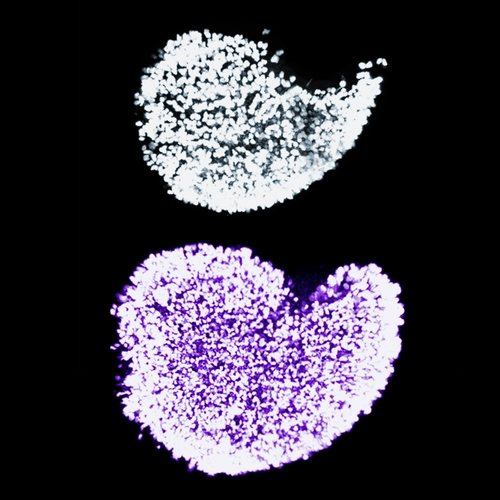
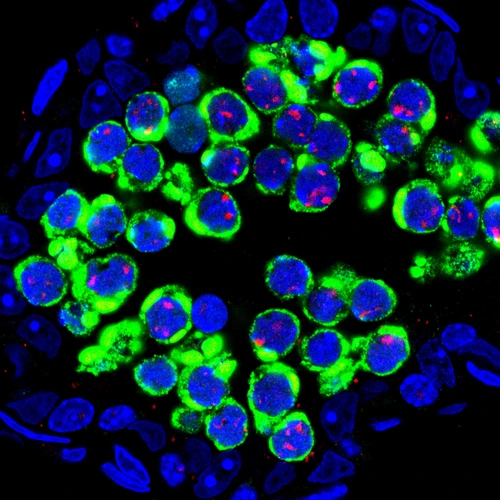
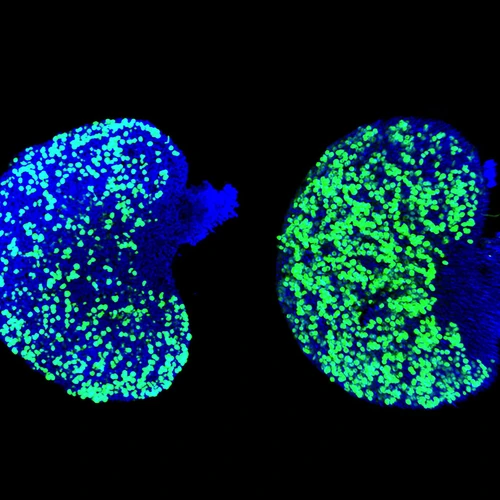
Integrity of hereditary material — the genome — is critical for species survival. Genomes need protection from agents that can cause mutations affecting DNA coding, regulatory functions, and duplication during cell division. DNA sequences called transposons, or jumping genes (discovered by Carnegie’s Barbara McClintock), can multiply and randomly jump around the genome and cause mutations.
Research
The primary goal of our research is to understand the development and differentiation of mammalian germ cells. We approach this problem by focusing on the complex interactions of germ cells with transposable elements (transposons, TEs) populating their genomes.
Transposons are discrete DNA sequences that can multiply and spread around the genome. They were first discovered in the 1940s by a Nobel Prize Laureate Barbara McClintock who was a scientist at the Carnegie Department of Genetics. You might enjoy watching her 1983 Nobel Lecture. It is not like any of your TED talks of today, but it gives a terrific insight into the mind of an incredible scientist.
TEs come in two flavors – DNA transposons and retrotransposons. TEs influence their host genomes in many different ways such as by perturbing genomic integrity and gene expression. And yet, numerous studies of plant, fungal and animal species uncovered overwhelming evidence of TE contribution to the evolution and divergence of genomes. A generally accepted view in the field is that TEs and their hosts are constantly involved in an arms race where each party tries to outwit and conquer the other. Given the critical role of germ cells for reproduction and propagation of species, their genomes represent the most attractive playground for TE activity. We posit that closer examination of the billion years-long battle between germ cells and TEs might provide new insights into the biology of both germ cells and TEs that could not be readily identified otherwise.
In our work, we focus on retrotransposon LINE-1 as it is the only autonomous element that still remains active in human cells. LINE-1 is a retrotransposon, which means that it moves from one location to another via an RNA intermediate. TE expression and activity are prevented by the combination of epigenetic and posttranscriptional mechanisms. These include repressive histone modifications, KRAB-zinc finger proteins, and small RNAs.
An important role in TE control in animal germ cells belongs to Piwi-interacting RNAs (piRNAs). Genetic studies in flies, worms, and mice showed that this class of small RNAs is an ancient adaptive mechanism of TE regulation. Since their discovery in 2006, much has been learned about piRNA production and functions. Our laboratory has also contributed to this field by studying a conserved gene Maelstrom (Mael) that is required for piRNA function in flies and mice. In addition, we have carefully analyzed phenotypes of Mael mutant mice to understand how LINE-1 activity impacts germ cells of both sexes. We frequently use an excellent antibody developed by Sandy Martin against LINE-1-encoded protein ORF1p to identify temporal windows of LINE-1 expression during germ cell development in wild-type and mutant animals. This reagent also allowed us to describe cytoplasmic nuage particles of mouse germ cells and to perform quantification of LINE-1 expression in individual germ cell nuclei.
The combination of the above approaches allowed us to uncover:
- An essential role of nuage germinal cytoplasmic structures in TE control.
- The appearance of DNA damage in male germ cells upon TE derepression.
- The presence of DNA damage in wild-type fetal oocytes.
- A critical role of differential LINE-1 expression in the selective elimination of wild-type fetal oocytes.
- Potential cytotoxicity of the intermediates of LINE-1 retrotransposition.
- Detrimental effects of LINE-1 overexpression on meiotic chromosome synapsis.
- A role for MAEL protein in primary piRNA biogenesis in male germ cells.
- Unique RNA-binding properties of an HMG-box domain of MAEL protein.
- Transient relaxation of DNA methylation at the onset of meiosis.
Current Topics
We approach this problem through the analysis of the function of Maelstrom, a conserved protein implicated in transposon silencing in flies and mice by means of specialized small RNAs known as Piwi-interacting RNAs (or piRNAs).
We posit that the never-ending battle between the genome and selfish elements has profoundly influenced germ cell biology in the course of evolution. We are trying to understand how LINE-1 elements impact germ cells in the course of their normal development and differentiation, particularly during meiosis.