Stanford, CA—Everyone who took high school biology learned that photosynthesis is the process by which plants, algae and select bacteria transform the Sun's energy into chemical energy during the daytime. But these photosynthetic organisms activate other biochemical pathways at night, when they generate energy by breaking down the sugars, starches, and oils that they created during the day. New work that focused on this nighttime growth found a protein that is necessary for it to occur and, surprisingly, the role of this protein was linked to the construction of the plant’s cellular membranes. It is published by Proceedings of the National Academy of Sciences.
The paper is from an integrated, international group of plant science experts, led by Carnegie's Arthur Grossman and including lead author Wenqiang Yang and co-author Martin Jonikas, both from Carnegie. It demonstrates that in the photosynthetic alga Chlamydomonas, the protein ferredoxin-5 is critical for growth in dark and for proper membrane organization.
Ferredoxin-5 is a member of a family of small iron-sulfur proteins that provide the electrons needed to drive various metabolic reactions in the cell. Photosynthetic organisms typically have multiple different types of ferredoxins, although little is known about how their functions differ.
Chlamydomonas has several ferredoxins, including the ferredoxin-5 protein described in this study. The work presented suggests that the ferredoxin variants, including ferredoxin-5, may each have specialized metabolic roles in the cell.
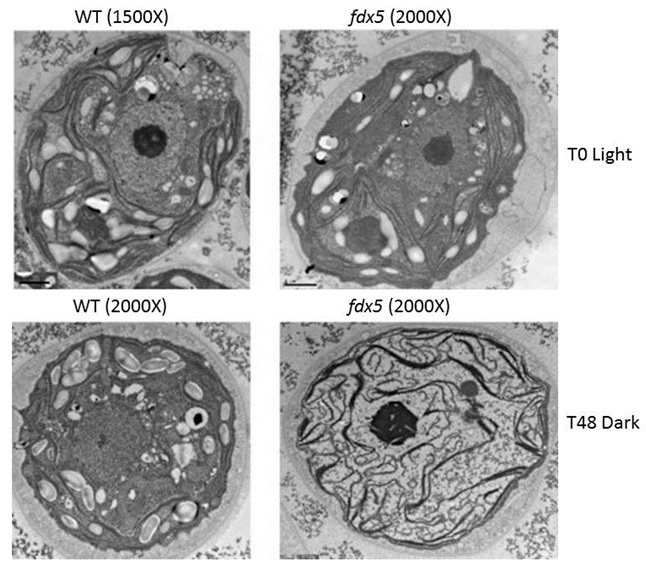
The team found that a specific mutant lacking the ferredoxin-5 protein was unable to grow in the dark (in the nighttime) or perform normal metabolic functions, but it had no trouble growing during the light (in the daytime).
As it turns out, the mutant alga also exhibited aberrant organization of cellular membranes when maintained in the dark, but did not exhibit them when maintained in the light.
These membranes are made up of lipids, which are fatty acids linked to a glycerol backbone. The ferredoxin-5 mutant shows marked alterations in the bond structure of many of these fatty acids, which changes the degree of saturation in the fatty acids present in the membranes. (Fully saturated fatty acids have single bonds between all of their carbon atoms while unsaturated fatty acids may have anywhere from one to several double bonds between their carbon atoms.) This, in turn, appears to impact the lipid composition of the membranes and results in aberrant membrane structure that impairs metabolic processes, including photosynthesis, that occur on those membranes.
The team’s findings suggest that one of the biochemical reactions that ferredoxin-5 drives through its electron-donating abilities is this alteration in the saturation state of fatty acids. This result indicates that when the alga is in the dark, ferredoxin-5 specifically donates electrons to enzymes that specialize in desaturating fatty acids; it may also donate electrons to other processes in the dark. (In the light, however, another member of the ferredoxin family may take on this responsibility, which is why the aberrant membrane structure is only observed in plants that are maintained in darkness.) .
"This work homed in on the role of ferredoxin-5 and factors critical for photosynthetic organisms to switch between daytime and nighttime metabolism," Grossman said.
This manuscript represents the efforts of a number of researchers in many different fields. Other co-authors include Matthew Posewitz at the Colorado School of Mines, Christoph Benning of Michigan State University, Sabeeha Merchant of the University of California, Los Angeles, Alexandra Dubini from the National Renewable Energy Laboratory and Xenie Johnson and Jean Alric of CEA/CNRS in Cadarache, France.
Top image caption: Nalca leaf in Chile, photo by Carnegie President Matthew Scott.
__________________
This work was supported by the Office of Biological and Environmental Research, GTL Program, Office of Science, U.S. Department of Energy; the National Science foundation; the National Institutes of Health; MSU AgBioResearch; the Carnegie Institution for Science; and the Royal Thai Government Scholarship.
