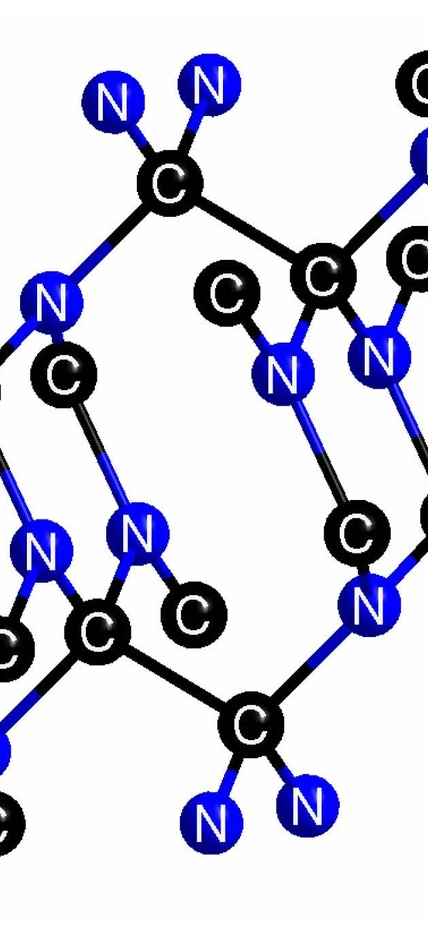
Washington, DC— New work from a team led by Carnegie’s Alexander Goncharov has created a new extremely incompressible carbon nitride compound. They say it could be the prototype for a whole new family of superhard materials, due to the unexpected ratio of carbon and nitrogen atoms. Their work is published in the journal Chemistry of Materials.
Compounds that are made up of carbon and nitrogen are of great interest to materials scientists, because they can be superhard and very resistant to heat. It’s predicted that some chemical structures of carbon and nitrogen could even be harder than diamonds! If such carbon nitrides were synthesized, they could have a number of practical applications, particularly in the arenas of materials machining and protective coatings. But producing them in actuality has been elusive.
“It was long ago theorized that a certain structural form of carbon nitride composed of three carbons and four nitrogens would create the ultimate harder-than-diamond material, the Holy Grail of materials science,” Goncharov explained.
Synthesis of some superhard materials with this 3:4 ratio of the two components been reported this way, but the results are often disputed. In fact, some scientists even suggested that this proposed ratio was less than ideal for creating a material with the desired hardness.
So Goncharov and his team set out to use synthesize a new ultra-incompressible form of carbon nitride in very clean, pure conditions with just the component elements under intense pressure and high temperatures. Their carbon and nitrogen atoms were under more than 550,000 times normal atmospheric pressure and heated to more than 12,000 degrees Fahrenheit.
What they created was a compound with a 1:1 ratio rather than the long-sought-after 3:4 ratio. The material satisfies all the conditions for superhardness, including a high number of bonds with very short distances between the component atoms. It is also not metallic, meaning it isn’t capable of conducting the flow of electrons that makes up an electric current. This is important, since metallic compounds are less hard than insulating ones, which don’t conduct electricity well.
“This work demonstrates that a good understanding of the laws of high pressure chemistry is crucial to discovering new chemical structures never seen under ambient conditions,” Goncharov said.
“We will see many more such novel materials with unusual compositions in near future,” stated Elissaios Stavrou, formerly of Carnegie and now at the Lawrence Livermore National Laboratory.
The paper’s other authors are Sergey Lobanov of Carnegie, Huafeng Dong and Artem Oganov of the Stony Brook University, Vitali Prakapenka of the University of Chicago, and Zuzana Konôpková of DESY Photon Science.
Top Image Caption: An illustration of chemical structures showing how elemental carbon and nitrogen under extreme pressures and temperature conditions (more than 550,000 times normal atmospheric pressure and heated to more than 12,000 degrees Fahrenheit) were synthesized into this new incompressible form of carbon nitride. Artwork is provided courtesy of Elissaios Stavrou.
___________________
This work was supported by DARPA, the National Natural Science Foundation of China, the Deep Carbon Observatory, the Chinese Academy of Sciences, and the Ministry of Education and Science of the Russian Federation.
Part of the work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Security. GSECARS is supported by the U.S. NSF and DOE Geosciences. Use of the APS was supported by the DOE-BES.