Introduction and history by Andrew Steele
Astrobiology is the interdisciplinary study of two of the broadest and most fundamental questions in human history: Are we alone? Where did we come from? Such fundamental questions require broad cooperation across science disciplines as progress in any one of these questions is not the sole provenance of a specific scientific field.
Carnegie scientists have tackled fundamental science questions applicable to astrobiology almost since its inception. So, when NASA sent out its call for astrobiology proposals in 1996, we were ready. Scientists such as George Cody, Marilyn Fogel, Nabil Boctor, and Robert Hazen were already working on origin of life chemistry and designing experiments to unpack the reactions that led to the formation of life on Earth. They were ideally positioned to secure the funding that kicked off the NASA Astrobiological Institute (NAI) and soon became one of the main three nodes of the research program. NAI officially ended in 2019, though work continues on campus.
Some of the work done on campus during that time:
- George Cody and postdoc at the time and later staff associate James Scott probed the limits of microbial life on Earth, essential research to understand the limits of life on any planet.
- Astronomers Alan Boss, Alycia Weinberger, and John Chambers unlocked the fundamental process of planetary formation and how our Solar System formed.
- Paul Butler’s work developing planet detection techniques with iodine cell doppler spectroscopy was fundamental to discovering the first extra-solar planets
- Sara Seager defined the search for signs of biological influence in the atmospheres of extra-solar planets.
- Yingwei Fei unlocked the deep dark depths of planetary interior processes that enable an environment to allow life to start and thrive.
- Larry Nittler, Conel Alexander, and George Cody studied how carbon moved through a forming solar system to become a fundamental building block for life
- Isotope geochemists Steve Shirey, Erik Hauri, and Rick Carlson used extremely high precision isotopic measurements to define the ages of meteorites, the Moon, and the Earth as well as how fundamental processes such as plate tectonics and volcanic eruptions shaped the Earth.
- Robert Hazen pioneered the concept of mineral evolution—linking an explosion in mineral diversity to the rise of life on Earth.
- Dionysis Foustoukos broke new ground in high pressure and temperature microbiology by integrating established experimental approaches with culture-based microbial studies to decipher microbial metabolic pathways.
All of this work, though it spans from the tracing of individual atoms to the physics of entire Solar Systems, comes together to form a fuller picture of the history of life on our planet, in our Solar System, and in our Universe.
Taking biology to space
Laboratory, observatory, and computer studies comprise only one side of how astrobiology questions are addressed on campus. Our astronomers utilize our observatories on the ground in Chile and reach into space using the Hubble space telescope (and soon the James Webb Space Telescope.) However, observation cannot take the place of getting “boots on the ground” to retrieve and study actual samples. That’s where space missions come in.
Sean Solomon, at the time the Director of the Department for Terrestrial Magnetism (DTM: now a part of the Earth and Planets Laboratory) and principal investigator of Carnegie’s astrobiology program, along with Larry Nittler conceptualized, built, launched, and managed the science of the MESSENGER mission to Mercury. The mission characterized Mercury’s surface composition, revealed its geologic history, revealed details of its magnetic field, and verified that it held water-ice at the poles.
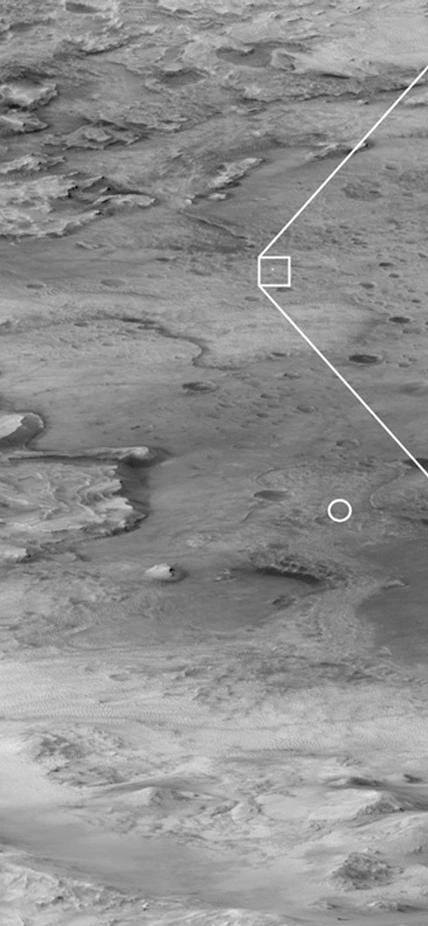
Caption: Hanging from a parachute about the Martian landscape, NASA’s Perseverance rover can be seen falling through the Martian atmosphere. Courtesy of NASA/JPL.
While Mercury may not seem like an obvious target for astrobiology because of its inhospitable surface, understanding the characteristics and development of the other planets in our Solar System is key to understanding why life started and was uniquely sustained on Earth. This was a point often made by former DTM director George Weatherill, whose pioneering work—including involvement in the earlier Mariner mission to Mercury—helped conceptualize our modern understanding of Solar System formation.
Carnegie scientists, Cody, Alexander, Nittler, and Andrew Steele were integral members of the science team studying samples from Stardust—a sample return mission from Comet Wild 2. Similarly, Nittler and Alexander studied samples from the Hayabusa 1 mission to asteroid 25143 Itokawa. (As of writing, Nittler is studying samples from Hayabusa II’s mission to the asteroid Ryugu.) These samples, while tiny, help tell the story of our Galaxy—revealing insights into the creation of our planet and of life itself.
On the biotechnical side of things, Steele’s program of instrument development and testing led to the first instrument to do automated detection of single bacteria in space, which flew to the International Space Station (ISS) in 2007. His team continued to build on this space flight instrumentation and developed and tested sample return prototype scoops, drilling, and coring systems that ultimately flew to Mars onboard Curiosity and Perseverance. They are now awaiting launch on the ExoMars missions.
These missions allow us to collect actual samples and provide essential data about our Solar system and our place in it.
Looking forward
The NASA Astrobiology Institute officially ended in 2019, but that doesn’t mean our pursuit of astrobiological questions has ended. In fact, it seems more and more likely that astrobiology is part of much of the research we do here on campus.
Our scientist’s involvement in missions continues with active participation in Curiosity, Hubble, James Webb Space Telescope, Perseverance, ExoMars, Hayabusa 2, and possible missions to Enceladus. Our astronomers are eagerly awaiting the building and commissioning of the Giant Magellan telescope—the next generation of super-large telescope that will revolutionize Earth-based observation.
Our scientists continue to work in fields that are astrobiologically relevant and continue to be funded by NASA and other funding agencies such as Sloan and Templeton to develop and test hypotheses with state of the art instrumentation on Earth with robotic spacecraft in our solar system and by observing distant planetary systems and processes elsewhere in our galaxy.
Below, are just a few examples of the questions we’re furthering on the Earth and Planets Laboratory campus.
Five key astrobiology questions we're exploring on campus:
1) Is there life elsewhere in our Solar System?

Caption: Katy Cain carefully holds Andrew Steele's sample (courtesy of David Blake) of the meteorite SAU 008. A sample of the same meteorite has now traveled back to Mars as a part of the calibration target for the SHERLOC spectrometer onboard the Perseverance rover. Image courtesy Carnegie Institution for Science/Katy Cain
While we’re fairly certain we won’t meet any sentient aliens in our Solar System any time soon, we do think that there may be things like microbes populating places as close as our neighbor Mars. The trick may be as simple as knowing what to look for.
Staff scientist Andrew Steele has worked his whole career at Carnegie searching for signs of life in meteorites from Mars and refining and defining protocols to detect an unknown microbe on Mars, in ancient Earth samples, and elsewhere in the Solar System. This has led to the discovery of novel ways organic molecules are synthesized on Mars from the study of Mars meteorites and led to Steele discovering these molecules on Mars as part of the Sample Analysis at Mars team onboard the Curiosity rover and Mars being declared the first habitable world outside of Earth.
“Robust life detection of an unknown alien micro-organism whose biochemistry may be completely different to terrestrial life has been a goal of my research for over two decades” says Steele.
From the analysis of the Martian meteorite ALH 84001, which was purported to contain signs of ancient Martian life to samples on the surface of Mars, Steele has continued to define the protocols and procedures necessary to detect a possible martian organism. “The trick is to use as few assumptions as possible,” says Steele, which has led to the development of what he likes to call the LMNOP principle.
He makes three basic assumptions about alien life
- Life is carbon-based.
- Organic chemistry is universal.
(i.e. The abiotic (non-life) building blocks that led to life are made throughout the solar system.) - Life uses only a small subset of these molecules.
(e.g. the distribution of amino acids, life tends to use only a small subset of the possible amino acids that are synthesized by abiotic processes)
“Think of it this way,” says Steele, “the genetic code on Earth is comprised of five letters—thymine (T), cytosine (C), adenine (A), guanine (G), and uracil (U). Abiotic synthesis creates a much larger series of letters and numbers, but life chooses just these five.”
Steele is looking for chemistry that is similarly concentrated in this peculiar fashion, which can not be explained by organic chemistry alone. This would be a sign of life. However, it might not be ACTGU like in terrestrial life, but rather LMNOP—totally different from Earth life
“If you see this, then bingo,” says Steele, “that is something highly indicative of a biosignature of extraterrestrial microbes.”
Once discovered, scientists would “go to town” on those samples, hitting them with every technique to try to uncover the reason for that concentration of letters and to seek what other signs—e.g. fossil cells or isotope distributions—may indicate life. According to Steele, it is crucial that a “null hypothesis” is adhered to—if you want to find life, assume there is no life and disprove that hypothesis. This leads to a robust and statistically significant approach to life detection.
2) How did Earth give rise to life? (And how does life impact Earth’s evolution?)

The transition from a chemical world to a biological one remains a profound mystery.
We know that Earth’s early atmosphere was low in oxygen and high in methane. It wasn’t a world where multi-cellular life could thrive, but we know that methane-eating microbes arose fairly early in our planet’s history. In fact, based on chemical evidence, scientists think microbes probably arose somewhere around 3.7 billion years ago—for comparison, our planet is 4.54 billion years old.
What we want to know is what happened in that pre-biotic time period that led to the development of those early microbes? How did early life impact our planet in return? And can we expect to see a similar development pattern for life on other planets? This is called the abiotic/prebiotic/biotic transition.
One way we’re studying this question is by blurring the boundary between biology, geophysics, and mineralogy. Carnegie scientist Robert Hazen pioneered the concept of mineral evolution—linking an explosion in mineral diversity to the rise of life on Earth.
Hazen said, “It turns out that about half of all the minerals on Earth form because of the biosphere, and this was a revelation to the field of mineralogy. We think about minerals, playing a role in forming the first cell and supporting life, but it works the other way around too.”
According to Hazen’s evolutionary theory of mineralogy, Earth’s minerals environment progressed in ten stages as our planet developed and the environment changed. (We’re in stage 10 now, by the way.) Each stage precedes the other, and the idea is that all planets may have to go through these or similar stages to develop and sustain life. This kind of evolutionary structure will come in handy for our exoplanet astronomers who are looking for signs of life on other planets.
Another promising area of this research is to investigate Earth’s natural catalysts and the environments in which they are found.
George Cody and colleagues study catalytic properties of transition metal minerals that are abundant in deep-sea ore-bodies to help piece together the puzzle of life’s origins. Similarly, staff member Andrew Steele has uncovered an electrochemically driven organic synthesis mechanism in operation on Mars, and is applying this reaction to any planet where volcanic rocks contact seawater. At the same time, Carnegie researcher Shaunna Morrison is working on the ENIGMA project, which aims to study the emergence and evolution of protein catalysts—and how Earth’s geology played a role in their evolution.
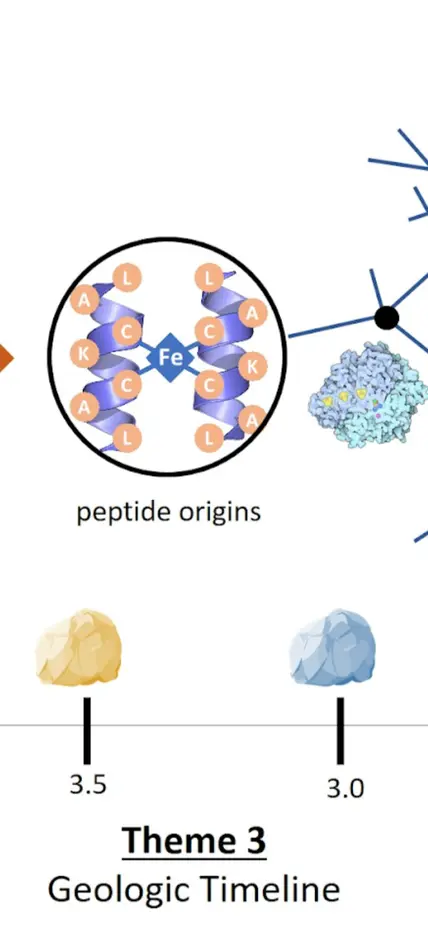
Caption: One project that addresses this question is the ENIGMA project is split into three themes. Two focus on the development of proteins as nanomachines. Carnegie's Shaunna Morrison is a part of the team that is exploring how proteins and the geosphere co-evolved through geologic time. Credit: ENIGMA
3) Where did Earth’s carbon come from?

Credit: Fragments of the Tarda carbonaceous chondrite meteorite that fell in Morocco in 2020 and that has a similar appearance to the returned Ryugu samples. Studying samples like this from meteorites help scientists build a timeline of our Solar System’s development. Courtesy Carnegie Institution for Science/Katy Cain
Wondering about where we came from has occupied the human imagination since the dawn of consciousness. Using samples from comets and meteorites, George Cody tracks the element carbon as it moves from the interstellar medium, through Solar System formation, ultimately to the origin of life.
Primitive meteorites, interplanetary dust particles, and comets are remnants of the early Solar System. The abundant organic matter contained in these primitive bodies records a long chemical history, beginning with reactions that occurred in the interstellar medium, and continuing with reactions that occurred during the formation and evolution of the early solar nebula, and in the formation and evolution of the parent bodies of meteorites. To untangle this record is a challenge: the vast majority of the organic carbon exists as an extremely complex polymer—large molecules with repeating units—that is insoluble by most means.
Cody and colleagues pioneered procedures applying solid-state nuclear magnetic resonance (NMR) spectroscopy to get around the insolubility problem. NMR spectroscopy reveals molecular information when nuclei of certain atoms are placed in an enormous magnetic field and then resonantly excited with radio-frequency pulses. The emission signal from the excited nuclei yields a spectral “fingerprint” characteristic of the electronic structure of the host molecule.
Cody also employs Carbon X-ray Absorption Edge Structure spectroscopy, which is essential to the analysis of comet particles. Results from both methods ultimately provide essential clues regarding the origin of extraterrestrial organic carbon and the history of chemical processing as the molecular cloud coalesced into the Solar System.
The retention of carbon in the inner Earth is a prerequisite to the origin of the global carbon cycle. Cody with colleagues has conducted NMR-based experiments that reveal how some carbon was retained even during the magma-ocean phase of Earth history. Such carbon may have been essential for the emergence of life.
4) How can we detect life on exoplanets?
Thanks to the sheer number of exoplanets that have been discovered, the ease with which models tell us planets organize themselves into orbits around stars, and recent leaps forward in our knowledge of the Milky Way’s chemical evolution, today’s scientists are fairly certain we will find life (or a planet that could host life) out there somewhere—if we know where to look.
We can’t look at a planet light-years away to physically see if it has tectonic activity. So what can we learn about a planet by studying them through modern telescopes and instruments?
For one, we can generally tell if a planet is in the habitable zone—where liquid surface water can exist—by measuring the distance to its host star and figuring out its surface temperature from there. But, being in the habitable zone is far from the whole story.
We can also determine a planet’s mass by seeing how it interacts with its host star. Stars and their planets are locked in a gravitational dance in which they are both pushing and pulling on each other. This means even a small planet causes a slight wobble in its host star that we can measure and use to work out its mass. Once we know a planet's mass and radius, we can figure out its density and determine if it’s rocky, gaseous, or metallic. Because Earth is our only source of comparison, scientists assume that habitable planets should be rocky.
Then there’s the atmosphere.
When we find a planet we want to study, we look at the starlight that permeates and interacts with the planet’s atmosphere. Each chemical in the atmosphere gives off or absorbs a signature wavelength of light. As the light makes its way to our telescopes, we use an instrument called a spectrograph to break it apart—creating a colorful spectral fingerprint that astronomers decipher to tell us what makes up the atmosphere.
In these spectra, scientists can detect things like water, carbon dioxide, and methane that could be signs of life. They can also use the atmospheric composition to get a glimpse into the planet’s internal processes—since the planet is what’s building and sustaining the atmosphere.
That means scientists have to get really good at reading spectra.
One multi-disciplined and multi-institutional project that is addressing this question is the AEThER project, which aims to define atmospheric signals of abiotic planets in order to rule out false positives.
Anat Shahar, who is the principal investigator on the AEThER project, explains, “In our lifetime, the most likely place we will be able to see signs of life outside of our Solar System is in the atmosphere of another planet.”
5) What are the limits to life on our planet and elsewhere?
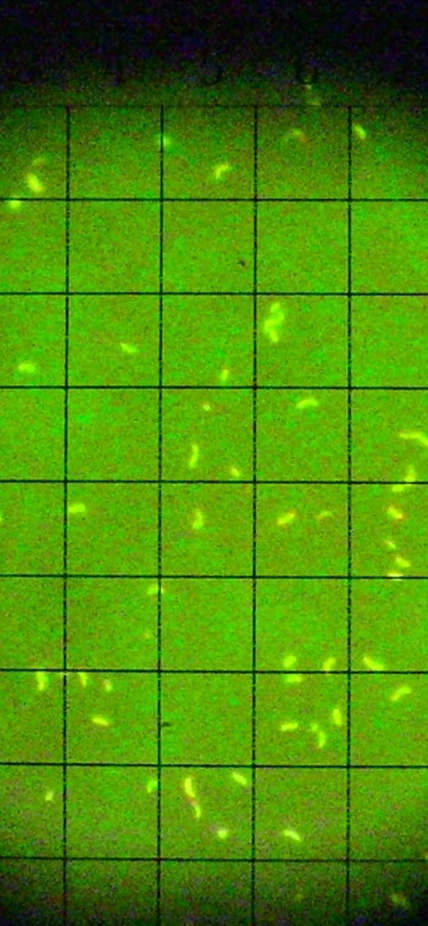
Credit: Stained microorganisms collected from deep-sea vents at 2.5 km depth and cultured at high-pressure conditions (250 atm) using innovative high-pressure microbiology techniques developed at the Earth and Planets Laboratory. This was the first time that microorganisms from deep-sea submarine volcanoes have been sampled, transferred, and cultured under such high-pressure and high-temperature conditions. Image courtesy of Carnegie Institution for Science and Dionysis Foustoukos.
The Earth and Planets Laboratory is known for its high-pressure labs for studying minerals under the intense temperatures and pressures found inside of planets. But can we use also use heat and pressure to study life that thrives in extreme conditions? The answer is yes!
Understanding the evolution and function of microbes in extreme conditions, like that of Earth’s deep-sea hydrothermal vents, can help us understand the adaptations of microorganisms to life in extreme conditions on other planets. So we put the pressure on in the lab.
For example, one of the most exciting discoveries related to the search of life on other planets is that Enceladus, one of Saturn’s moons, releases plumes of hydrogen from its surface, suggesting ongoing hydrothermal activity and thermodynamic disequilibrium in its subsurface, ice-covered ocean [Waite et al., 2017]. The implication for extraterrestrial life is that microbes that can use inorganic chemicals and minerals like hydrogen, sulfur, and nitrogen for energy, might inhabit this deep alien ocean.
Carnegie’s Dionysis Foustoukos uses high-pressure microbiology techniques developed on campus to study the metabolism of Earth’s own deep-sea microbes in both pure cultures and in their natural deep-sea communities. To do this, he subjects them to the intense temperatures and pressures found at seafloor and subseafloor environments.
Some of the key questions his team is looking at are: How do high-pressure microbes adapt to the pressure and chemical gradients present in deep-sea and subsurface environments? Which are the core cellular functions for pressure adaptation? Do all high-pressure microbes have the same adaptations to help them survive these extreme environments?
In these studies, they investigate the pressure and metabolic adaptation mechanisms of these microbes. Recently, Foustokos and colleagues followed elemental sulfur through microbial metabolism, while microorganisms are growing at seafloor pressures.
“We also want to learn how microbially-derived volatiles—like carbon dioxide and water—are introduced, transported, and stored in the atmosphere, hydrosphere, and the interior of terrestrial-like planets,” says Foustokos. “These features are profoundly linked to habitability, the origin of life, and the observable evidence of conditions favorable for life.”
These science questions and the continuing pursuit of answers to the fundamental questions of “Are we alone?”, “Where did we come from?” and, “Where are we going?” continue to thrive at Carnegie and this is exemplified in each of the Carnegie Institution for Science’s divisions: “Earth”, “Life” and “Space”.