Stanford, CA—Photosynthesis is probably the most well-known aspect of plant biochemistry. It enables plants, algae, and select bacteria to transform the energy from sunlight during the daytime into chemical energy in the form of sugars and starches (as well as oils and proteins), and it involves taking in carbon dioxide from the air and releasing oxygen derived from water molecules. Photosynthetic organisms undergo other types of biochemical reactions at night, when they generate energy by breaking down those sugars and starches that were stored during the day.
Cells often face low-oxygen conditions at night, when there’s no photosynthesis releasing oxygen into the air and all photosynthetic and non-photosynthetic organisms in the environment are respiring oxygen.
When this happens, some organisms such as the single-cell alga Chlamydomonas are able to generate cellular energy from the breakdown of sugars without taking up oxygen. They do this using a variety of fermentation pathways, similar to those used by yeast to create alcohol. Although critical to the survival of common aquatic and terrestrial organisms that are found all over the planet, many of the details regarding this low-oxygen energy creation process are poorly understood.
New work from a team including Carnegie’s Wenqiang Yang and Arthur Grossman, and in collaboration with Matt Posewitz at the Colorado School of Mines, hones in on the biochemical pathways underlying the special flexibility of Chlamydomonas in responding to oxygen-free and low-oxygen conditions. Other Carnegie co-authors include Claudia Catalanotti, Tyler Wittkopp, Luke Mackinder, and Martin Jonikas. It is published by The Plant Cell.
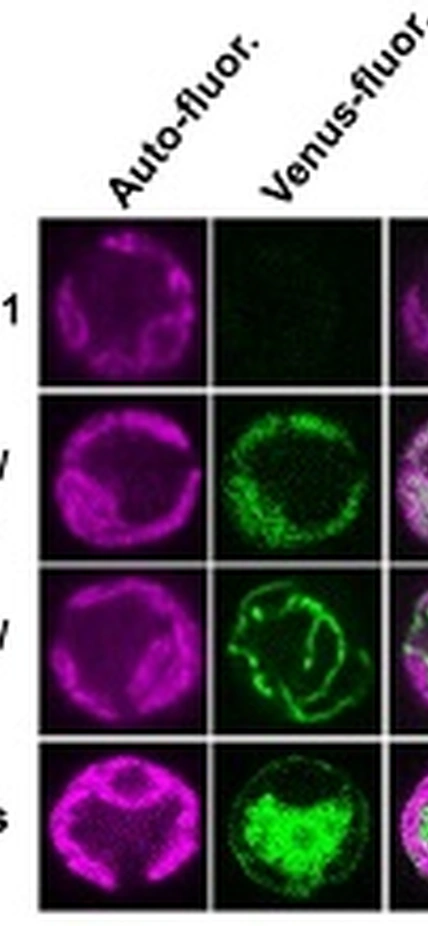
In an arduous and exacting step-by-step process, the team used a series of specially created mutants to determine the importance of two identical branches of the fermentation pathway that are located in different compartments in the cell, both believed to be essential to dark, low-oxygen fermentation in Chlamydomonas. The pathways are dependent on four proteins, PAT1 and PAT2 and ACK1 and ACK2.
ACK1 and PAT2 are located in a part of the plant cell called the chloroplast, which is the compartment where photosynthesis takes place. ACK2 and PAT1 are located in the mitochondria, the organelle in plant and animal cells where sugar breakdown takes place.
“Surprisingly, we found that the chloroplast pathway is much more critical than the mitochondrial pathway for sustaining fermentation metabolism, even though generating energy from the breaking down of sugars is generally considered a mitochondrial process,” Grossman said.
What’s more, they found that although the PAT- and ACK-controlled pathways are indeed crucial to generating energy under these conditions, and to producing an important metabolite called acetate, there appear to be other undiscovered biochemical pathways that are participating in this process as well and capable of picking up at least some of the slack in the system.
“The system needs more work, especially if we are going to understand the ways in which the day-night cycle and environmental oxygen levels impact the productivity of photosynthetic organisms on our planet,” Grossman added.
Image Caption: Localization of ACK and PAT Venus fusion proteins in Chlamydomonas. Picture courtesy of Wenqiang Yang.
__________________
This work was funded by the Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, US Department of Energy grants, the National Science Foundation Award, and South Carolina Experiment Station project. It is designated as technical contribution of the Clemson University Experiment Station.