Stanford, CA— Like humans, plants are surrounded by and closely associated with microbes. The majority of these microbes are beneficial, but some can cause devastating disease. Maintaining the balance between them is critical. Plants feed these microbes, and it’s thought that they do so just enough to allow the good ones to grow and to prevent the bad ones from gaining strength. This system of microbe feeding is mediated by proteins called sugar transporters.
Carnegie’s Wolf Frommer previously identified a unique class of sugar-transport proteins, called SWEETs, which play key roles in plants such as producing nectar, exporting energy manufactured in the leaves to the other plant organs, and filling seeds with nutrients to feed a plant embryo.
In two separate papers, he and several teams of researchers unravel the molecular structure of SWEET2, a transport protein that plays a critical role in limiting the sugar supply available to root microbes to the right level. This is the first time the structure of a member of the SWEET class of proteins has been described, and only one of three structures elucidated so far for sugar transporters in animals and plants.
One team, led by the Stanford University School of Medicine’s Liang Feng, an assistant professor of molecular and cellular physiology, elucidated the molecular structure of a SWEET2 transporter from rice. Discovering the structure of SWEET2, combined with determining key amino acids in the protein necessary for function, is the key to figuring out the mechanism by which it works. This is important for understanding what happens when the transporter fails due to disease or pathogens and for learning how to protect against these risks. Their work is published October 19 by Nature.
Frommer and Feng had previously worked together to determine the structure and mechanism of the bacterial analog to SWEET transporters, called SemiSWEETs. It has been predicted that SWEETs arose by a doubling and fusion of the bacterial version of the transporter genes during evolution. The similarities they found between the structural folds of the SemiSWEET dimer (a complex of two identical units that associate) and SWEET2, combined with their evidence that SWEET2 likely functions as part of a complex built of cooperating units of individual proteins, strongly support this theory.
The other team—on which Frommer worked with Carnegie postdoc Woei-Jiun Guo (now at National Cheng Kung University) and Dorothea Tholl of Virginia Tech—focused on SWEET2’s role in protecting the mustard plant Arabidopsis from parasitic infection. They show that SWEET2 helps stockpile sugars in a storage bubble inside of plant cells called the vacuole, thereby limiting the sugar supply intended to feed only the good microbes to a level that prevents the growth of the bad guys. This work has been recently published online by The Plant Journal.
Plants secrete sugar into the soil immediately surrounding their roots. Although the molecular mechanisms for this are poorly understood, it is thought that the sugar is a reward for symbiotic microorganisms living in this region that facilitate the plant’s growth. However, this sugar can also be taken up by pathogens, such as the parasite Pythium. Pythium is what’s called an oomycete, similar to a fungus, which is responsible for root-rot and other diseases in field and greenhouse crops.
The research team showed that SWEET2 facilitates the retention of sugar in roots, which could be a mechanism of starving and resisting pathogens living in the immediate root surroundings. They found that SWEET2 expression was increased 10-fold during Pythium infection and that specially created mutants lacking SWEET2 were more susceptible to the parasite.
“Together, these two papers provide first insights not only into how plants control carbon sequestration into the soil, but also improve our understanding of the functioning of this unique class of SWEET transporters, including the important human SWEET homolog,” Frommer said.
Stanford’s Liang Feng remarked: “This research is an important step in understanding the sugar transport process and energy homeostasis in cells.”
Other authors on the Nature paper include: lead author Yuyong Tao, as well as co-authors Shuo Li and Yan Xu of the Stanford School of Medicine; Carnegie’s Lily Cheung, Joon-Seob Eom, and Li-Qing Chen; and Kay Perry of APS and Cornell University.
Other authors on The Plant Journal paper include lead author Hsin-Yi Chen, as well as co-authors Ya-Chi Yu and Li-Hsuan Ho of National Cheng Kung University; Carnegie’s Li-Qing Chen; and Jung-Hyun Hug of Virginia Polytechnic Institute and State University.
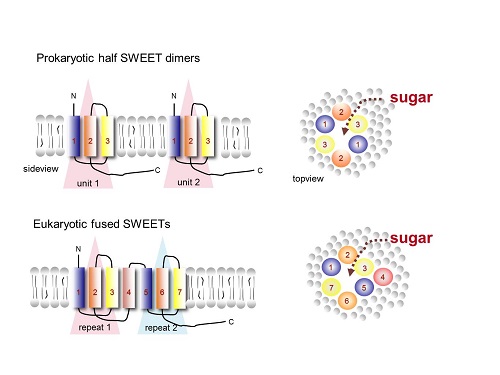
Caption: On the top left is a side view of the two identical bacterial SemiSWEET units, which together form the transport pore shown on the top right. On the bottom left is a side view of the plant sugar transporter SWEET2, in which the two units were fused via an additional helix (#4) to form a similar pore as in the SemiSWEET dimer from only a single protein, as shown in the bottom right.
__________________
The Nature paper was supported by Stanford University, the Harold and Leila Y. Mathers Charitable Foundation, the Alfred P. Sloan Foundation, National Natural Science Foundation of China, the Division of Chemical Sciences Geosciences and Biosciences Office of Basic Energy Sciences at the US Department of Energy, the National Science Foundation, and the National Science Foundation Postdoctoral Research Fellowship in Biology. Part of this work was based upon research conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team, which are supported by a grant from the National Institute of General Medical Sciences from the National Institutes of Health. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) and the Office of Science by Argonne National Laboratory, was supported by the U.S. DOE.
The Plant Journal paper was supported by the Ministry of Science and Technology Taiwan, the Office of Basic Energy Sciences of the US Department of Energy, and the National Science Foundation.