Washington, DC—If you freeze any liquid fast enough, even liquid metal, it becomes a glass. Vitrified metals, or metallic glasses, are at the frontier of materials science research. They have been made by rapidly cooling alloys of various metals including, zirconium, palladium, iron, titanium, copper, and magnesium, and used for a variety of applications from making golf clubs to aerospace construction. But much about them remains poorly understood.
A team including Carnegie’s Qiaoshi “Charles” Zeng and Ho-kwang “Dave” Mao, among others, is trying to figure out the rules that govern metallic glass’s creation. They are doing this by looking at metallic glasses under extreme pressures. High-pressure research can be used to probe structure on an atomic level and understand a material’s state of order or disorder.
Crystals are structured in repeating patterns that extend in every direction. Glasses lack this kind of strict organization. Crystalline metals often have weaknesses at the boundaries between crystal grains. Metallic glasses lack these defects, which makes them stronger.
From a practical standpoint, metallic glasses are extremely strong, hard, and resistant to wear and corrosion, all of which make them good potential candidates for engineering uses, including electronics casings, and medical uses such as surgical pins and stents. But so far, their creation is a time-consuming and expensive process, because scientists lack a general metallic glass theory. Discovering fundamental rules governing metallic glasses, in order to guide their development, could be revolutionary.
The team—which also included Carnegie’s Zhidan Zeng, Stanislav Sinogeikin, Yoshio Kono, Curtis Kenney-Benson, Changyong Park, and Wenge Yang—probed glass made from alloys of the metal cerium under pressure. High-pressure research teams at Carnegie have probed cerium alloys extensively, so it was a natural fit for this research. Their goal was to look for fundamental rules correlating the structure and properties of metallic glasses that could aid their further discovery and synthesis going forward. Their findings are published by Proceedings of the National Academy of Sciences.
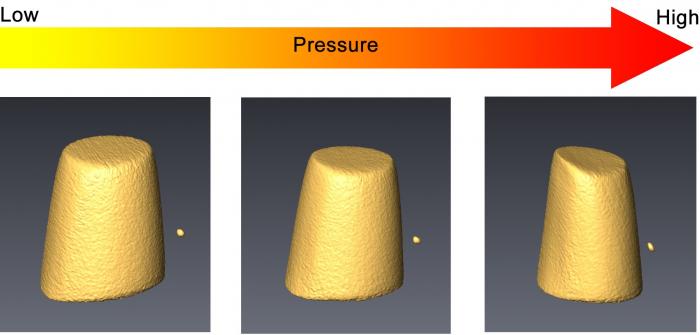
“High pressure compression is an extremely powerful tool for fine-tuning both the structures and the properties of materials such as metallic glasses and for looking for information that is consistent across samples,” said lead author Zeng. “By accurately measuring the evolution of structure and properties during compression, we could establish a relationship between them. The more we learn about the structure-property relationship in metallic glass, the better we can predict the properties of other potential metallic glasses.”
Through their work on cerium-based metallic glass, the team was able to discover a consistent rule that establishes an exact numeric relationship between the decrease in volume of metallic glass under pressure in relation to changes in its atomic structure determined using advanced X-ray tools. This numeric relationship held for metallic glasses at ambient pressure, too.
“The exactness and universality of this rule we’ve discovered indicates that the atomic structures of metallic glasses are not totally random, even if they aren’t as regularly packed as crystalline metals; they may share some predictable structural features. This is an important step in understanding what controls the formation of these materials” Zeng said.
_________________
This work was supported by the DOE, the NSF, and the National Natural Science Foundation of China.