Baltimore MD— We would not expect a baby to join a team or participate in social situations that require sophisticated communication. Yet, most developmental biologists have assumed that young cells, only recently born from stem cells and known as “progenitors,” are already competent at inter-communication with other cells.
New research from Carnegie’s Allan Spradling and postdoctoral fellow Ming-Chia Lee shows that infant cells have to go through a developmental process that involves specific genes before they can take part in the group interactions that underlie normal cellular development and keep our tissues functioning smoothly. The existence of a childhood state where cells cannot communicate fully has potentially important implications for our understanding of how gene activity on chromosomes changes both during normal development and in cancerous cells. The work is published in Genes and Development.
The way that the molecules that package a cell’s chromosomes are organized in order to control gene activity is known as the cell’s “epigenetic state.” The epigenetic state is fundamental to understanding Spradling and Lee’s findings. To developmental biologists, changes in this epigenetic state ultimately explain how the cell’s properties are altered during tissue maturation.
“In short, acquired epigenetic changes in a developing cell are reminiscent of the learned changes the brain undergoes during childhood,” Spradling explained. “Just as it remains difficult to map exactly what happens in a child’s brain as it learns, it is still very difficult to accurately measure epigenetic changes during cellular development. Not enough cells can usually be obtained that are at precisely the same stage for scientists to map specific molecules at specific chromosomal locations.”
Lee and Spradling took advantage of the unsurpassed genetic tools available in the fruit fly to overcome these obstacles and provide new insight into the epigenetics of cellular development.
Using a variety of tools and techniques, they focused on cells in the fruit fly ovary and were able identify a specific gene called lsd1 that is needed for ovarian follicle progenitor cells to mature at their normal rate. The researchers found that the amount of the protein that is encoded by this gene, Lsd1, which is present in follicle progenitors decreases as the cells approach differentiation. What’s more, the onset of differentiation could be shifted by changing the levels of Lsd1 protein that are present. They deduced that differentiation ensues when Lsd1 levels fall below a critical threshold, and that this likely corresponds to when genes can be stably expressed.
“The timing of differentiation is very important for normal development,” Lee said. “Differentiation onset determines how many times progenitors divide, and even small perturbations in Lsd1 levels changed the number of follicle cells that were ultimately produced, which reduced ovarian function.”
Previously, it was thought that the follicle cell progenitors started to differentiate based on an external signal they received from another kind of ovarian cells known as germ cells. Lee and Spradling found that while this germ cell signal was essential, it was already being regularly sent even before the progenitors responded. Instead, it was the Lsd1-mediated change in their epigenetic state that timed when progenitor cells started to respond to the signal and begun differentiating. Once they become competent, however, differentiating follicle cells communicate extensively with their neighbors, and continued to do so throughout their lives.
As is frequently the case in basic biological research, the molecules and mechanisms studied here are found in most multicellular animals and hence the researchers conclusions are likely to apply broadly throughout the animal kingdom, including in humans.
In addition, to the importance of this research for understanding how animal chromosomes change during normal development, it may also help clarify alterations in the epigenetic state that take place in some cancers. A minority of cells in such cancers begin to express high levels of Lsd1 and to behave like undifferentiated progenitors.
“Studying fruit fly follicle cell differentiation can teach us at a deeper level what Lsd1 is doing in both normal and cancerous progenitors,” Lee added.
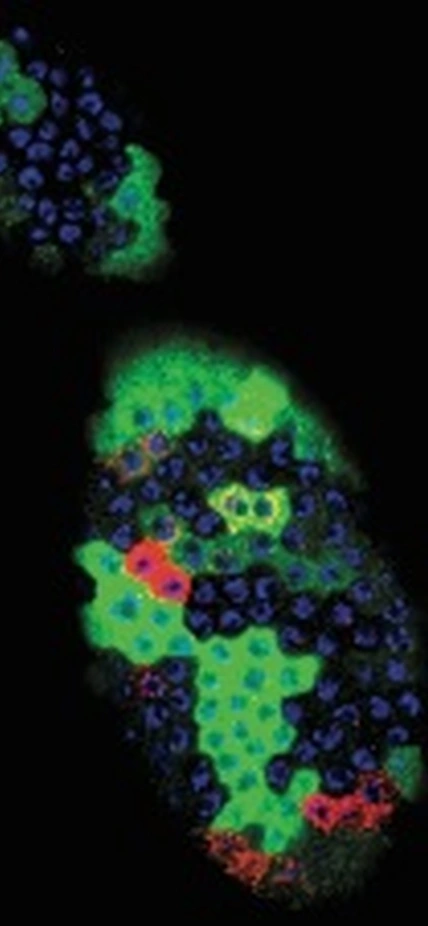
Image Caption: Fruit fly ovarian follicle progenitor cells, with different colors marking a specific kind of activity (red) specific gene expression (green) and nuclear DNA (blue).
__________________
This work was funded in part by the Howard Hughes Medical Institute.